Mobile contingents of the medical service and civil disaster control provide medical care in the event of an operation. The preclinical first aid at the place of illness, injury or wounding serves on the one hand to save life and preserve organs or functions, and on the other hand to make the patient fit for transport.
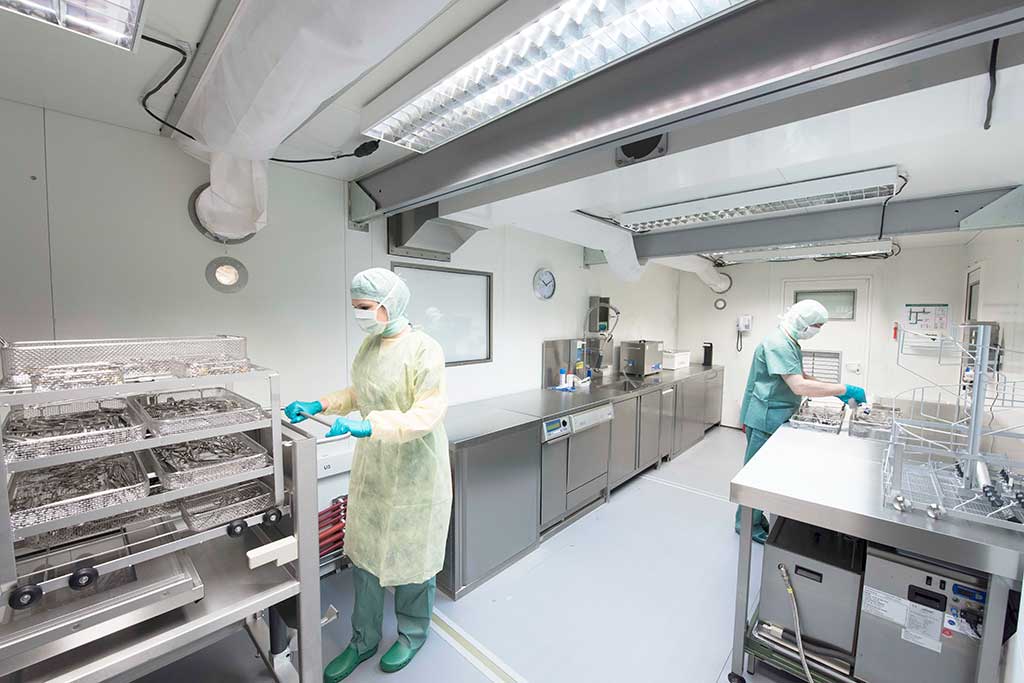
Comprehensive medical care is ensured by mobile hospitals that carry out operations. The prerequisite for efficiently working surgical modules are efficient central sterilisations that clean instruments quickly and gently with consistently high quality and provide them in a sterile state.
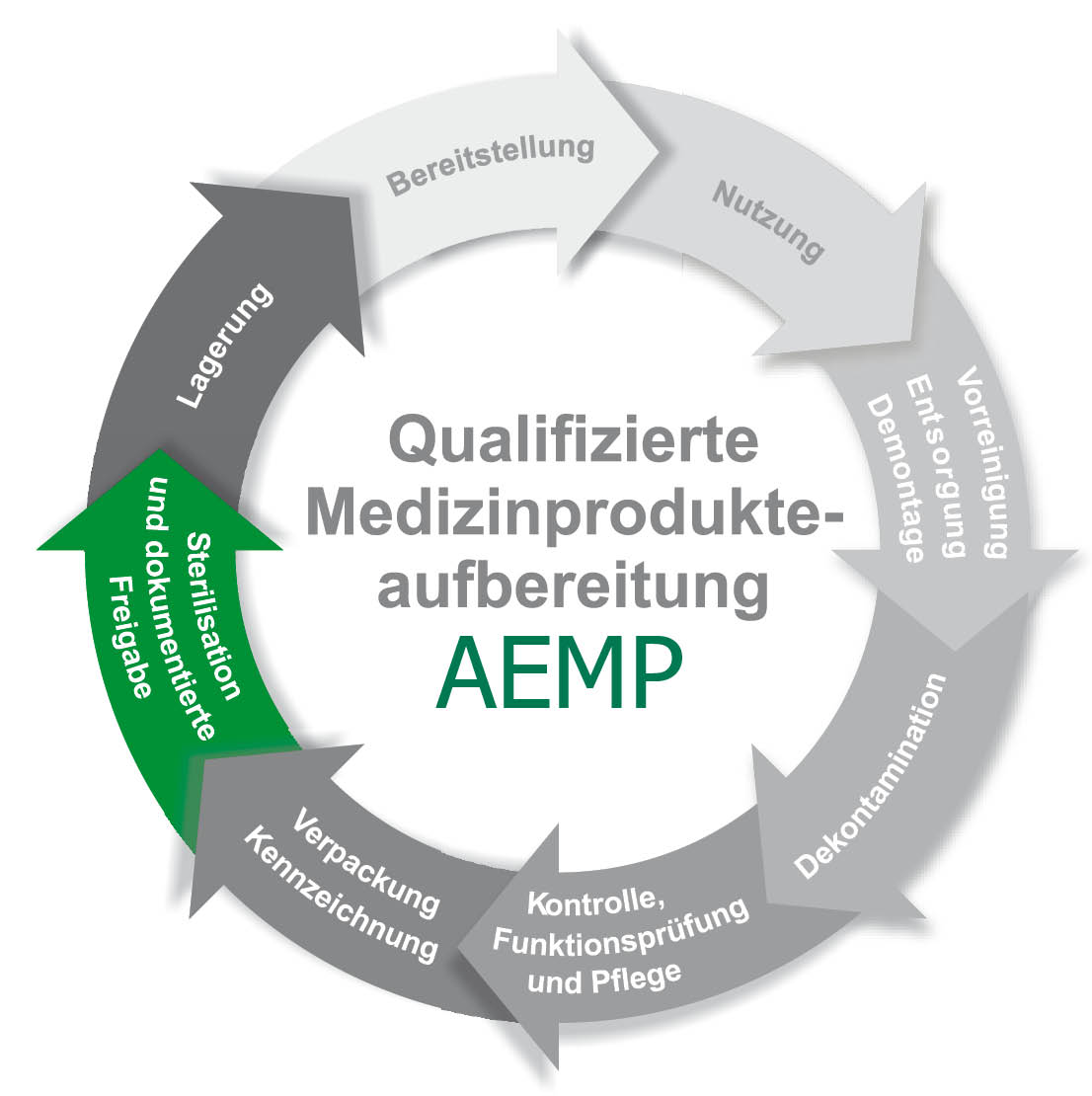
The requirements for instrument reprocessing or central sterilisation (CSSD) * have also changed significantly in mobile service. In the past, instruments were cleaned manually and then sterilised with steam. Today, the focus is on a reproducible, validatable cleaning process**, with unclean and clean working areas as well as crossing-free paths. This requires, for instance, mechanical rinsing processes with thermal disinfection and drying before these instruments are further processed in standard-compliant sterilisers. The process parameters relevant to the sterile items during cleaning and sterilisation must be recorded and archived.
* Central sterile supply department ** The recommendations of the Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM) on the “Hygiene requirements for medical devices” must be observed.